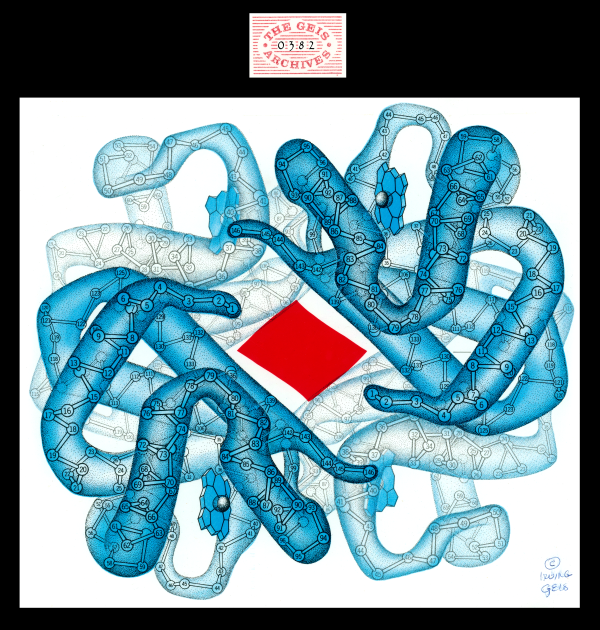
Deoxyhemoglobin
Unknown Year, Unknown Dimensions

Geis illustrates deoxyhemoglobin in blue, a color associated with the absence of the oxygen molecule. Hemoglobin undergoes a conformational change in the absence of oxygen to produce this conformer. In the center is a red area showing the footprint of the space between the beta chains in the oxygenated state. The beta chains move apart in the deoxygenated state.
Used with permission from the Howard Hughes Medical Institute (www.hhmi.org). All rights reserved.

Related PDB Entry: 4HHB
Experimental Structure Citation
Fermi, G., Perutz, M.F., Shaanan, B., Fourme, R. (1984) The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J.Mol.Biol. 175: 159-174.
About Hemoglobin
Hemoglobin is a protein found in the red blood cells of all vertebrates that is responsible for the transport of oxygen. The heme groups in the molecule produce a red color in arterial blood when oxygenated. Hemoglobin has a tetrameric quaternary structure of two alpha and two beta chains, each containing a ring-shaped heme group, giving the overall appearance of having four myoglobins combined into one structure. These heme groups use their central iron atom to bind oxygen, and, in this way, blood carries oxygen from the respiratory organs throughout the body. While hemoglobin is best known for its role in vertebrate respiration, it is also found in some invertebrates, fungi, and plants, where it transports other gases such as carbon monoxide, nitric oxide, and hydrogen sulfide.
Text References
Dutta, S. & Goodsell, D. (2003). Molecule of the Month: Hemoglobin. DOI: 10.2210/rcsb_pdb/mom_2003_5
Initial Structure Determination
Perutz, M. F., Rossmann, M. G., Cullis, A. F., Muirhead, H., & Will, G. (1960). Structure of hæmoglobin: a three-dimensional Fourier synthesis at 5.5-Å. resolution, obtained by X-ray analysis. Nature, 185, 416-422.