Zinc Transporter ZntB
July 2010

Careful Control
Zinc is a cellular paradox: it plays an essential role in cells, but free zinc ions are highly toxic, attacking cellular machinery. Because of this, zinc levels in the cell are carefully regulated, ensuring that there is just enough for the necessary structural and catalytic roles, but not enough to pose a danger. The level of zinc is controlled by a diverse collection of pumps and channels that ferry zinc ions in and out of the cell. Our genome contains at least two dozen zinc transporters to fit the needs of different types of cells.Funneling Zinc
The ZntB zinc transporter controls the flow of zinc out of bacterial cells. It is a funnel-shaped protein composed of five identical subunits, that together form a pore through the membrane. The structure of the intracellular domain was solved by researchers at MCSG, available in PDB entry 3ck6. The smaller portion that crosses the cell membrane was not included in the structure, and is shown here in lighter colors based on the similar magnesium transporter CorA, PDB entry 2iub.Structure and Function
The structure of ZntB revealed several functional features of the transporter. While solving the structure, five localized peaks of electron density were discovered in each of the subunits. After careful study using anomalous diffraction, it turned out that the peaks were not zinc ions, but rather, a collection of chloride ions. Analysis of electrostatics suggests that these chloride ions tune the properties of the funnel, neutralizing positively-charged amino acids just enough to favor passage of zinc ions rather than monovalent ions like sodium and potassium. The structure also revealed two rings of acidic amino acids at the base of the funnel, which may be important for stripping water molecules off of zinc ions before they are transported.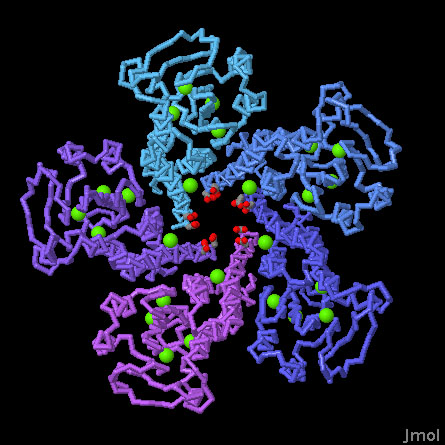
ZntB Zinc Transporter (PDB entries 3ck6)
The intracellular domain of the transporter is included in this structure. The chloride ions are shown in green and the two aspartate rings, aspartate 235 and 242, are shown with oxygen atoms in red. Use the buttons below to change between different representations of the protein, and click and drag the mouse in the window to rotate the molecule.
References
- Tan, K., Sather, A., Robertson, J. L., Moy, S., Roux, B. and Joachimiak, A. (2009) Structure and electrostatic property of cytoplasmic domain of ZntB transporter. Protein Science 18, 2043-2052.
- Eide, D. J. (2006) Zinc transporters and the cellular trafficking of zinc. Biochimica et Biophysica Acta 1762, 711-722.
- Berg, J. M. and Shi, Y. (1996) The galvanization of biology: a growing appreciation for the roles of zinc. Science 271, 1081-1085.



